nCAR-TTM Platforms
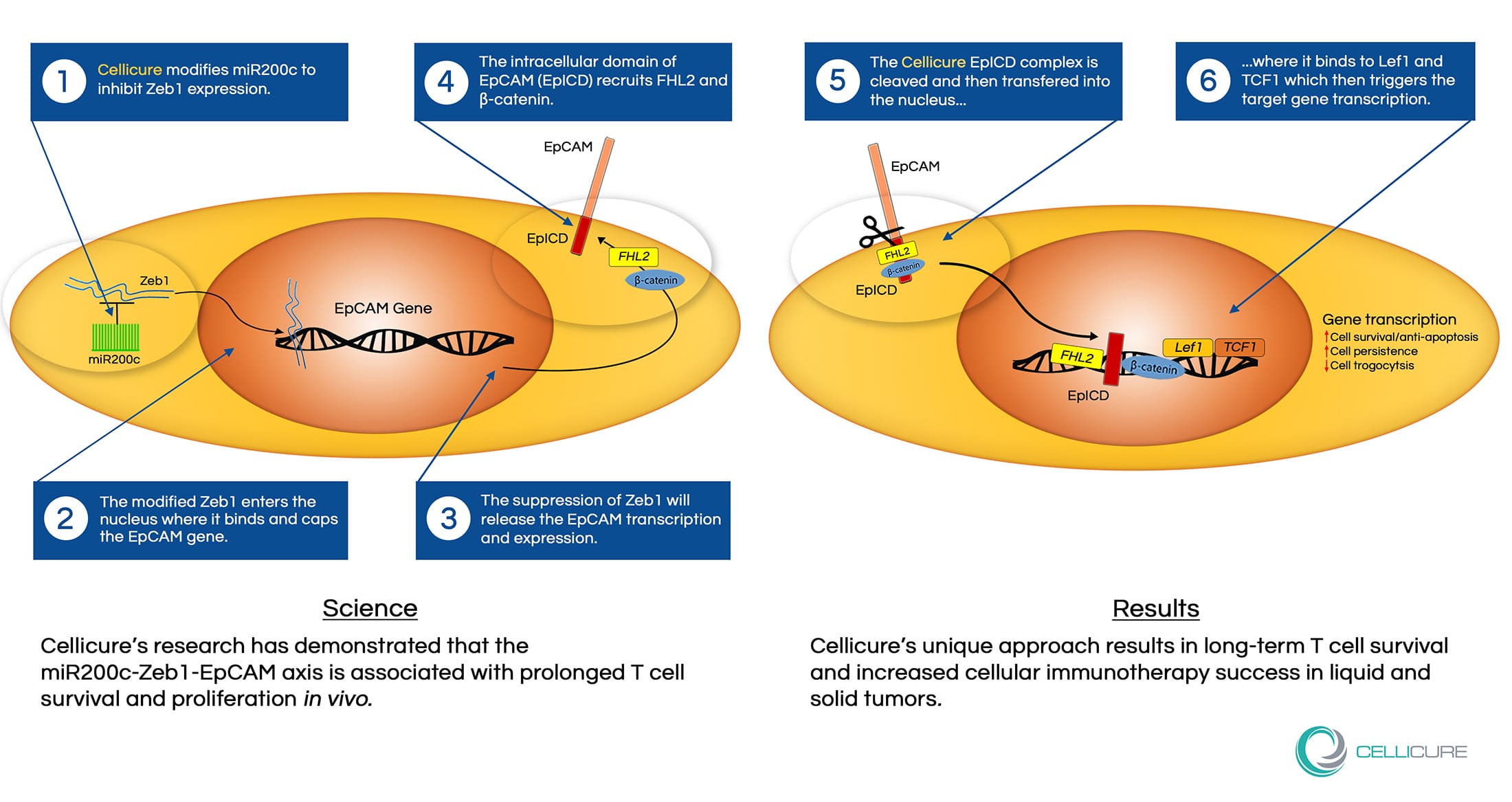
Genomic miRNA “Trogocytosis” screening and RNAseq and ATACseq analysis have demonstrated that the miR200c-Zeb1-EpCAM axis is associated with prolonged in vivo T cell presistence. miR200c suppresses the expression of Zeb1, a zinc finger and homeodomain transcription factor that binds, caps, and suppresses the EpCAM gene causing a decrease in EpCAM transcription.
Suppression of Zeb1 results in an increase in EpCAM transcription leading to the EpCAM intercellular domain recruitment of FHL2 and β-catenin. The recruitment then forms a complex that translocates to the nucleus with Lef1 and TCF1 triggering target gene expression. This process mediates cell survival leading to long-term cellular proliferation in vivo as well as improved T cell immunotherapy in liquid and solid tumors.